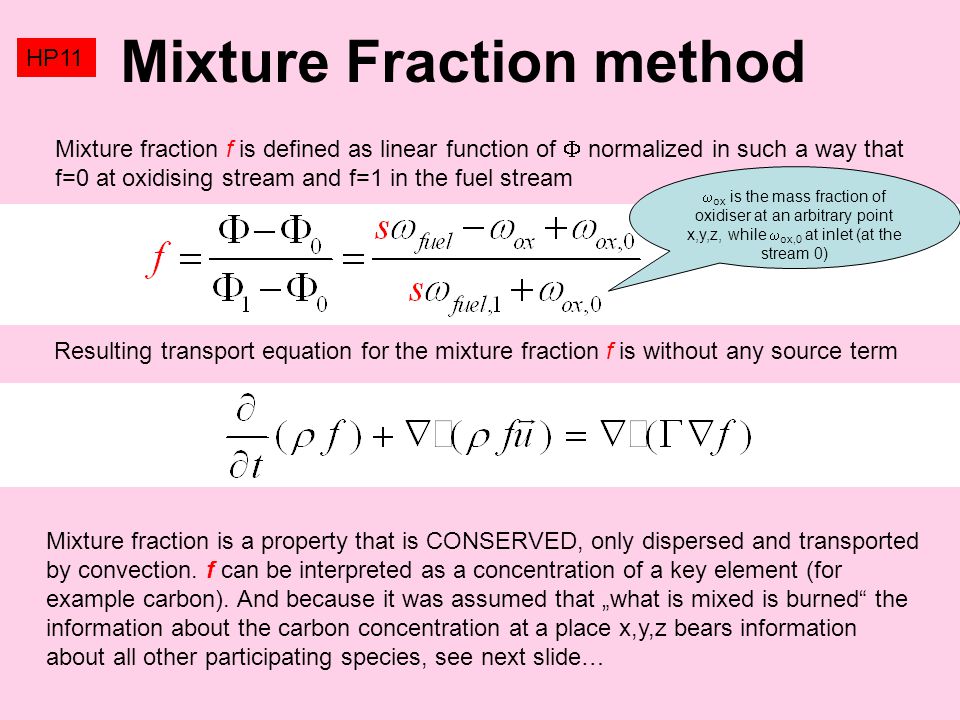
SOLVED: A gas mixture contains 1.25 g N2 and 0.85 g O2 in a 1.55-L container at 18 C. Calculate the mole fraction and partial pressure of each component in the gas mixture.

Mixture fraction analysis of combustion products in the upper layer of reduced-scale compartment fires - ScienceDirect

A mixture has 18 g water and 414 g ethanol. The mole fraction of water in mixture is (assume ideal behaviour of the mixture) :
Fundamentals Of Combustion (Part 1) Dr. D.P. Mishra Department of Aerospace Engineering Indian Institute of Technology, Kanpur L
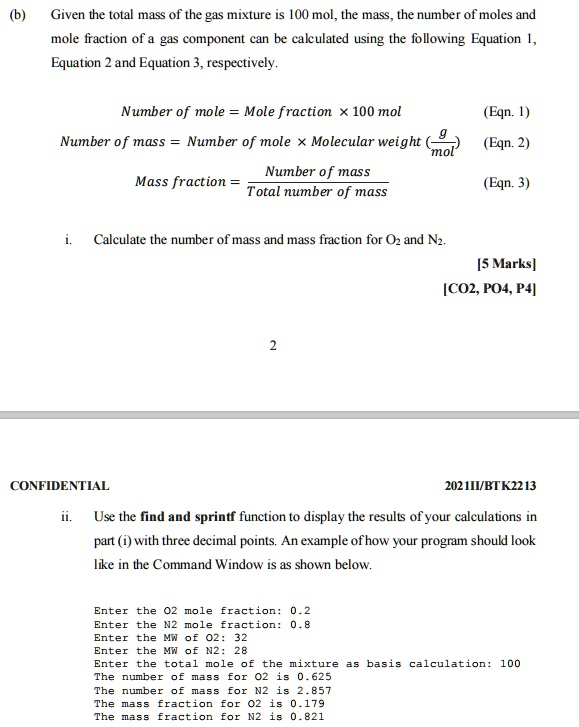
SOLVED: Given the total mass of the gas mixture 100 mol, the mass , the number of moles and mole faction of gas component can be cakulated using the following Equation 1,

Question Video: Determining the Mole Fraction of a Gas Given the Mole Fraction of the Other Gas in the Mixture | Nagwa